What unit is used to measure viscosity?
Pa·s (pascal-seconds) are used to indicate the viscosity of a fluid.
For example, when making a sponge cake, you might decorate it with whipped cream. Whipped cream can be given shape as it is pushed out of a piping bag or spread on the side using a spatula. However, once the shape is changed, it stays that way.
Then, what would happen if we used vegetable oil instead of whipped cream? You may be able to put it in a piping bag, but as you can imagine, you won’t be able to squeeze it out like cream, of course, and the oil will just pour out without maintaining its shape. The ability to maintain a shape—or lack thereof— is related to viscosity and whether or not the substance has thixotropic properties.
“Viscosity” refers to the “stickiness” of a liquid
“Viscosity” is a term often associated with adhesives. Viscosity is a quantitative measure of the stickiness of a liquid, with higher values indicating less flowability and lower values indicating more flowability.

Learning about the “viscosity” that surrounds us
Viscosity can be expressed using the international unit (SI unit) Pa·s (pascal-seconds). In practical use, a smaller unit, mPa·s (millipascal-seconds), is used. How viscous are the things around us?
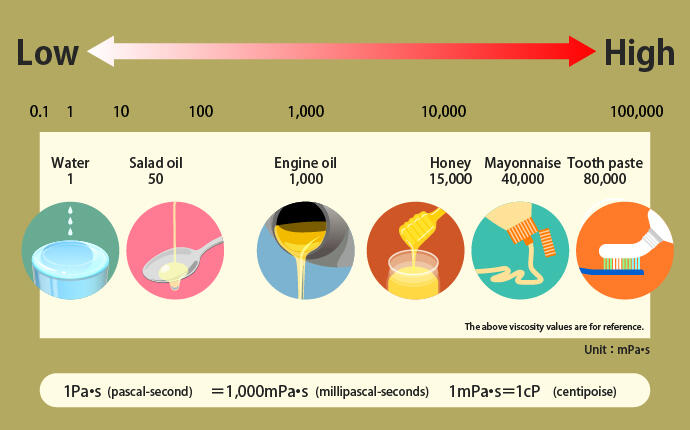
As a side note, 1 Pa·s (pascal-second) = 1,000 mPa·s (millipascal-seconds), and 1 mPa·s (millipascal-second) = 1 cP (centipoise).
You might be able to more-or-less recognize that the viscosity values of water and toothpaste are very different. The adhesives and sealants produced by ThreeBond also have “viscosity.” This is indicated in the catalogs and product information pages, so you can see that different products have different viscosity.
Now, there's another difference between water and toothpaste. It’s the difference in ability to maintain a shape.
Like the whipped cream discussed at the beginning, toothpaste can be pushed out of a tube and put on a toothbrush, and when you stop squeezing the tube, it will maintain its shape. If water and toothpaste are the same in that they have viscosity, what’s the difference between the two?

Liquids with thixotropic properties
When force is applied to liquids with the same viscosity, the difference in their ability to maintain a shape is related to whether or not the liquid is thixotropic.
Thixotropy is a phenomenon in which the fluidity of a liquid temporarily increases when the liquid is subject to stress, and when the fluidity is lost, the liquid returns to the original state. In technical terms, it is a reversible phenomenon in which the structure of liquid components break down and form again.

Thixotropy around us
The whipped cream discussed earlier is also a thixotropic liquid. It comes out when you push it out from a piping bag, and the squeezed out cream maintains its shape, allowing you to make beautiful decorations. The same goes for toothpaste and mayonnaise.
The gel ink used in ballpoint pens is also thixotropic. Force is applied to the gel ink due to the rotation of the ball, allowing us to write. However, if you move the tip of the pen away from the paper—that is, if you stop writing, the ink won't come out. You can write with a ballpoint pen because its ink has the property to flow when you are writing and to stop when you aren't.


We can see that the properties and characteristics of substances are applied in the things we normally encounter in our day-to-day. We at ThreeBond also adjust the viscosity and thixotropic properties of our products to fulfill the requirements of our customers when developing adhesives and sealants. When selecting a product, we recommend you to take a look at these figures as indicators of the properties of the product.
