Why do single-use heat packs get hot?
Because they release energy (heat) through a chemical reaction!
When going outside in the cold of winter, single-use heat pack are a must, aren’t they. A single-use heat pack gets warm by itself some time after it is opened. Do you know why that is?
A single-use heat pack get warm because its ingredients oxidize!?
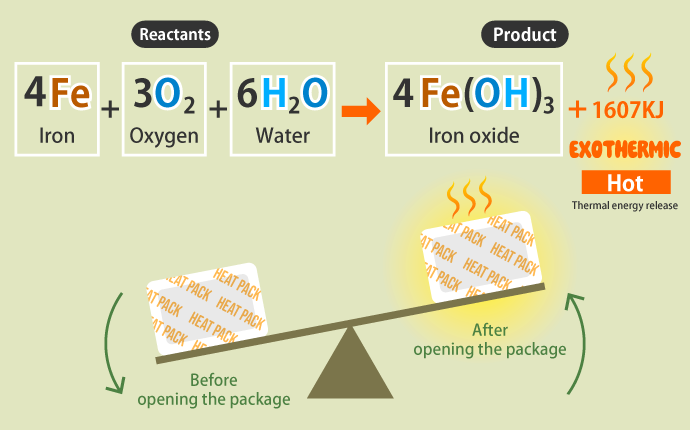
Single-use heat packs contain iron powder, water, and salts, to which vermiculite and activated charcoal are added as additives. Of these ingredients, iron powder is the substance that generates heat. Iron reacts with oxygen and moisture in the air to oxidize, generating heat in the process.
The water, salts, and vermiculite also contained in the packet serve to accelerate the oxidation speed of the iron, while the activated charcoal plays a role in keeping the oxidation speed of the iron in check so that the single-use heat pack keep its temperature for a long time, enabling it to maintain a comfortable temperature for an extended period.

We can say that single-use heat packs are products that make good use of the heat generated by a chemical reaction.
Exothermic and endothermic reactions by chemical reactions
Let's see how heat is produced by the oxidation of iron using a chemical formula.
The left side contains iron, oxygen, and water before oxidation (= reactants), and the right side contains oxidized iron (= product). When reactants change into a product, there is a difference in the chemical energy of the substances before and after the change. The difference in chemical energy transforms into heat energy and goes in or out, causing a change in temperature.
In the case of a single-use heat pack, the reactants before a packet is opened have a greater chemical energy than the oxidized product after the packet is opened, and thus heat energy was released. This phenomenon is called an exothermic reaction.
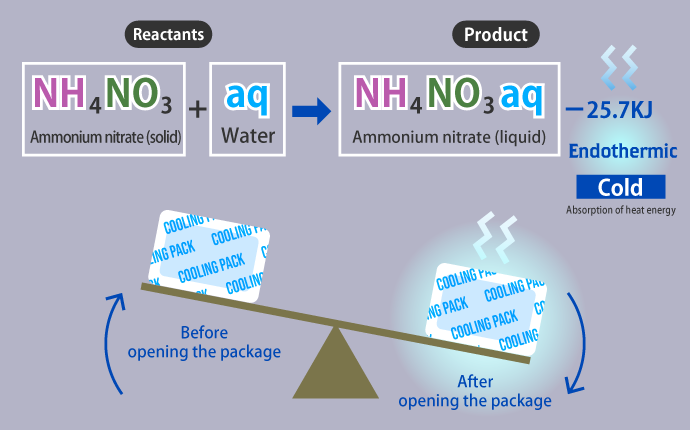
In contrast, an endothermic reaction is a phenomenon that occurs when the chemical energy of a product is greater than that of the reactants, which causes thermal energy in the surrounding area to be absorbed. We see cooling packs in stores in summer that use the endothermic reaction that occurs when ammonium nitrate reacts with water.
Can an exothermic reaction of adhesives keep you warm?
The mechanism that causes adhesives to solidify is also a "chemical reaction" of the substances contained in the adhesives. Therefore, exothermic or endothermic reactions may occur during solidification.
So, like a single-use heat pack, is it possible for us to get warm by utilizing the heat of the chemical reaction when the adhesive solidifies...?
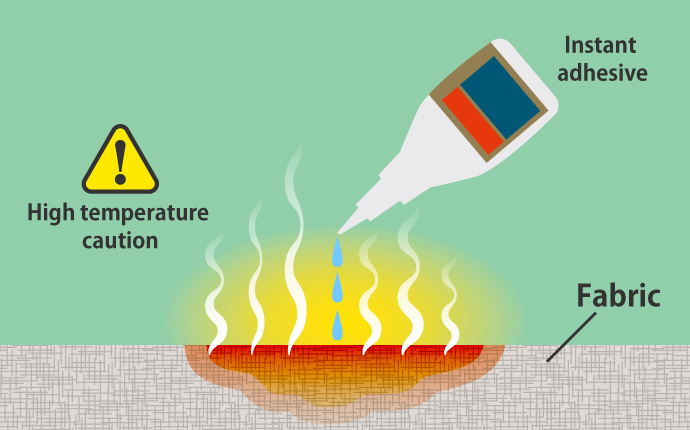
For example, instant adhesive, which is frequently used in daily life, reacts with moisture in the air to cure, and there is a large difference in energy before and after curing. There is no problem when using it on metal or plastic because the reaction occurs gradually. However, when it soaks into fabric, paper, or other fibers, and the surface area grows larger, the chemical reaction accelerates rapidly. In some cases, the heat of the chemical reaction may approach 100°C and is very dangerous.
In the same way, there are some other adhesives that require careful handling. Be sure to read the precautions before use.
Unfortunately, the heat of the chemical reaction of adhesives cannot keep you warm, but we hope that they can be of help when you make or repair something that comforts you.
